Jump to a section
General sample considerations
- Liquid chromatography (LC) samples should be non-volatile or semi-volatile samples. Volatile samples should be analyzed by gas chromatography (GC) instead. You can use the diagram below to help determine the best chromatographic method for your needs.
- Samples need to be dissolved in a suitable solvent at ca. 0.1 – 1 mg/mL.
- Solvent is dependent on separation type and stationary phase. Sample should be prepared in solvent similar to mobile phase A.
- Solvent choice depends on sample polarity, with more polar samples being dissolved in a more polar solvent (e.g., methanol) and a less polar sample being dissolved in a less polar solvent (e.g., hexanes). For samples with unknown polarity, an intermediate solvent, such as isopropanol or ethanol, may be more useful.
- High polarity solvents (e.g., methanol) should not be applied to strongly non-polar columns (e.g., normal phase separations), nor should highly non-polar solvents (e.g., hexanes) be applied to strongly polar LC columns (e.g., reversed phase separations).
- For best separation results, sample should be dissolved in mobile phase A, aka, the starting solvent/mobile phase, or a similar solvent.
- Generally, cleaner (i.e., higher grade) solvents contain fewer impurities that may interact with the polymer/sample material, interact with the column, or just complicate the analysis. HPLC grade solvents are preferred, but ACS grade is acceptable for samples.
- Samples are generally prepared at concentrations ca. 1 mg/mL or at 100 – 1000x dilutions. Peak shape is a strong indicator if higher or lower concentrations are needed. Broad or tailing peaks generally mean too much sample was injected and the sample should be diluted.
- Highly complex samples may benefit from additional sample preparation. Liquid (liquid phase extraction, LPE) or solid (solid phase extraction, SPE) extraction techniques can help clean and purify samples prior to analysis, reducing chromatographic complexity.
- Samples should be clear of precipitation, dust, or any other debris. Undissolved solids may require heat or sonication to assist in desolvation. Additionally, filtering may be useful in further eliminating particulates. For most LC/GC applications, a 0.22 μm filter is commonly applied.
- When working with a manual injector (i.e., no autosampler), the injector should be cleaned before and after use.
- A common cleaning solvent is isopropanol, which works for most samples.
- The injector should be rinsed with at least 2 loop volumes of 1 or 2 cleaning solvents.
- Before injecting sample, the loop should be flushed with mobile phase A. Otherwise, the residual isopropanol or other cleaning solvents may impact sample separation.

Column Chromatography
The most common types of column chromatography are normal and reversed phase chromatography; however, there are several variations of liquid chromatography. While the former methods separate components based on polarity, methods such as ion exchange and size exclusion (to name a couple) use ionic or size properties, respectively. Another important consideration in column chromatography is flow rate. Flow rate is primarily determined by column diameter and particle size. Analytical flow, for example, use columns with 1 – 5 mm inner diameters and typically have particle sizes of 2 – 5 µm to operate at ca. 0.2 – 1 mL/min.
Normal and reversed phase chromatography operate on a similar methodology. The stationary phase (i.e., column) and mobile phase are polar opposites (i.e., stationary phase is polar and mobile phase is non-polar, or vice versa). Sample components that have polarities more similar to the stationary phase, condense onto the column; whereas, sample components that have polarities more similar to the mobile phase, move through the column to be detected. The mobile phase polarity can also be adjusted to become more similar to the stationary phase, eluting each sample component in order of polarity, either increasing or decreasing.
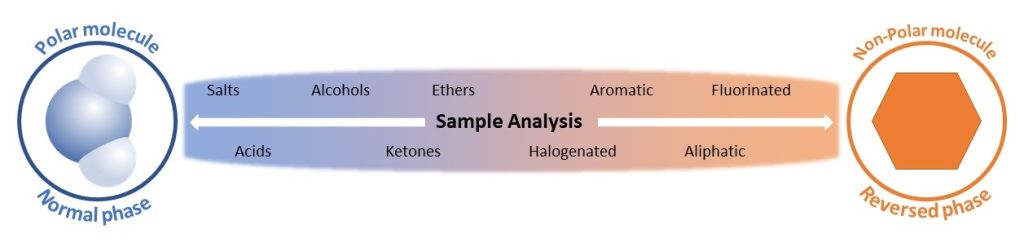
The sample components of interest determine if normal or reversed phase chromatography should be applied. Optimal separation is from components with polarities more similar to the stationary phase polarity. Normal phase stationary phases are polar, so polar samples will be more optimally separated. Reversed phase stationary phases are non-polar, so non-polar samples will be more optimally separated. Some functional group examples are shown below to help determine if normal or reversed phase separation would be more useful.
Experiments
There are two basic methods in column chromatography. In one method, a constant mobile phase or mobile phase combination is applied. This isocratic method uses a mobile phase with a constant polarity and separation is achieved via more diffusion methods. Finding a suitable mobile phase composition can take some time, but can help decrease method time as the column does not need to wash or equilibrate with every sample. This method is ideal for simple samples/experiments, high-throughout batches, or purification. However, clean samples with additional sample preparation (e.g., extractions), simple samples with few components, or short sample batches/sequences should be applied as the column can get very dirty or clogged, impacting separations. Since the mobile phase polarity is not changing, not all sample components will elute. After the sequence, the column should be extensively washed.
Another method, gradually changes the mobile phase composition over time. This gradient method, separates a wider range of components in a single method. The gradient begins at a polarity very different from the stationary phase and linearly changes to a polarity more similar to the stationary phase. Methods are referred to in terms of %B or %B/min. As %B is increased over time, the mobile phase polarity increases/decreases. Analytes elute in order of polarity, either decreasing or increasing polarity. Fast gradients (high %B/min) will elute analytes quickly but may sacrifice separation resolution. Separation can be improved by slowing the gradient (low %B/min). Generally, the resolution limit is 1%B/min, with little to no separation improvement being noted at slower gradients. Combining 2 to 3 gradients can help target specific analytes while still reducing the overall method time.
Additionally, gradient methods also involve a (1) washing step and (2) re-equilibration/regeneration step. Both of these steps should be considered in terms of column volumes. A column volume refers to how much solvent or time is needed to pass through the column. This can be calculated using cylinder volume (solvent amount), and time determined based on the flow rate.
Volume = (radius) x (π) x (length)
Washing should be performed for 1 – 2 column volumes to ensure all sample components have eluted prior to injecting a new sample. Regeneration prepares the column to separate a new sample. At least 3 column volumes are required to ensure each injection begins under the same conditions. In general, gradient methods look like the diagram below. Normal phase chromatography necessitates longer regeneration times as column equilibration is very slow.

Stationary Phase
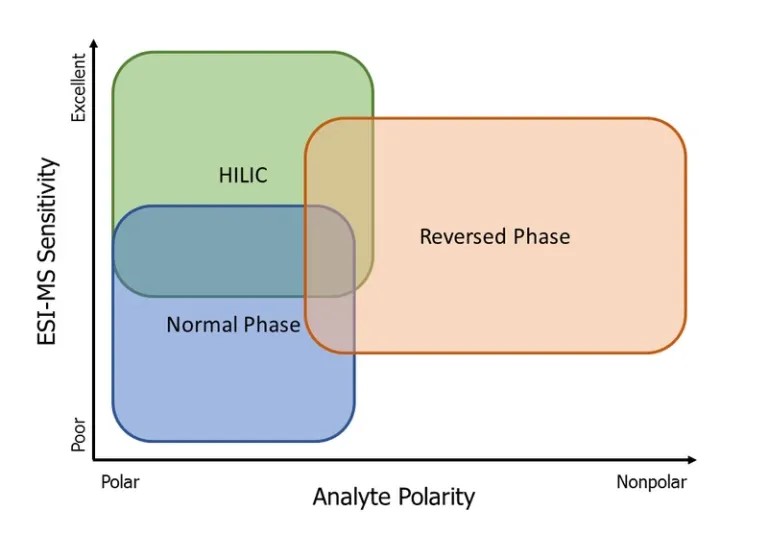
Stationary phase, more commonly referred to as the column, polarity greatly affects the optimal analytes to be separated. Column polarity determines what type of column chromatography will be performed, reversed phase (RP) or normal phase (NP). The image to the right shows a general representation of what polarity range each type of column chromatography optimally separates.
Column length is directly proportional to separation resolution. Longer columns produce greater theoretical plate heights (as described by Plate Theory), which can be related to how analytes partition between the stationary and mobile phases.
Reversed phase (RP): non-polar stationary phase and polar mobile phases. Analytes will elute in order of decreasing polarity (i.e., polar to non-polar constituents).
The stationary phase is non-polar and generally hydrocarbon chains of varying length (e.g., C4, C8, C18, C30). The most common RP column type is C18. As analyte size decreases, stationary phase chain length can be increased for improved separation. Large or bulky analytes may not be able to pass or easily pass through longer chain stationary phases. In this case, shorter stationary phase chains should be used. Hydrocarbon chains can also be encapped or embedded to modify the stationary phase polarity. Polar endcapping is useful for retaining more polar analytes better, thus improving their separation. Very polar analytes will be unretained on reversed phase columns and co-eluting in the void volume (i.e., the volume or retention time unaffected by stationary phase attractive forces) without separation.
Normal phase (NP): polar stationary phase and non-polar mobile phases. Analytes will elute in order of increasing polarity (i.e., non-polar to polar constituents).
The stationary phase is polar and generally silica or functionalized with cyano, diol, or amino groups. Very non-polar analytes will be unretained on normal phase columns and co-eluting in the void volume without separation. Because the stationary phase is polar, the presence of water in the sample or mobile phase can greatly affect retention times and reproducibility. Normal phase chromatography often suffers from poor reproducibility and long column regeneration times.
Hydrophilic interaction liquid chromatography (HILIC): a special type of separation where the stationary phase is polar and polar solvents. Analytes will elute in order of decreasing polarity (i.e., polar to non-polar constituents).
Sometimes referred to as a sub-catagory of normal phase chromatography because HILIC chromatography also uses polar stationary phases. Stationary phases are generally bonded amide groups. While normal phase mobile phases are 100% organic, HILIC mobile phases include water (< 20%). Like normal phase chromatography, HILIC suffers from similar reproducibility and regeneration weakness. However, by using polar mobile phases, HILIC is a suitable normal phase separation method compatible with mass spectrometric detection.
Mobile Phase
The mobile phase can consist of a single solvent or a combination of 2-4 solvents. Most pumps are binary, meaning only 2 mobile phase lines can run simultaneously. This means, if 3-4 solvent mixes are necessary for a chromatographic method, solution-based mobile phases will be required. For example, for a method needing water, acetonitrile, and isopropanol, mobile phase A might be 50/50 water/acetonitrile and mobile phase B might be 90/10 isopropanol/acetonitrile. Some pumps are quartenary, meaning 4 mobile phases can be used simultaneously. For the same example above, 3 separate lines of water, acetonitrile, and isopropanol could be used and the gradient adjusted to give the desired ratios.
Mobile phases can be pure solvents (e.g., 100% water), combined solvents (e.g., 60/40, v/v, acetonitrile/water), or buffers with added acid, base, and/or salts (e.g., water with 10 mM ammonium formate and 0.1% formic acid). The addition of acid, base, or salt can help increase separation resolution, sharpen chromatographic peaks, and/or increase detection. If acids and/or bases are added to mobile phases, pH should be carefully considered. Columns have a pH rating and operating outside that given pH range can destroy the stationary phase. The solvents applied to a chromatographic experiment depends on the stationary phase chosen. The most common mobile phases for each method is given in the table on the right.
| Column method | Mobile phase(s) |
| Reversed phase | Water, Methanol, Acetonitrile |
| Normal phase | Hexane, Chloroform |
| HILIC | Water, Acetonitrile |
It is also important to note solvent miscibility and boiling point. Immiscible mobile phase A and mobile phase B will not mix in the column, creating traveling solvent “bubbles” or boundary regions that will detrimentally affect sample separation and reduce reproducibility. Be sure all mobile phases and solvents are miscible before beginning any separation method. If changing a method, preparing the HPLC (changing columns, changing mobile phases, etc.), cleaning a column with immiscible solvents, be sure to rinse the lines or column with an intermediate, miscible solvent before changing to an immiscible solvent. For example, if switching from a reversed phase method/column to a normal phase method/column, the lines should be purged and flushed with the next mobile phase system before connecting the column. Additional purging and system flushing and/or purging/flushing with an intermediate solvent will be necessary. As another example, a common reversed phase cleaning method applies water, methanol, isopropanol, and dichloromethane in sequence to rinse off increasingly non-polar constituents; however, water and dichloromethane are not miscible but isopropanol and dichloromethanol are miscible. Therefore, after cleaning with water and methanol, the column should be thoroughly washed with isopropanol before cleaning with dichloromethane (in combination with isopropanol) and again washed thoroughly with isopropanol before switching back to water/methanol. For more column cleaning procedures, please see the SOP section.
Noting solvent/mobile phase boiling points are especially important if employing the column heater. The benefit of a column heater is to maintain the column at a stable temperature, either near room temperature or at an elevated temperature, thereby removing a variable. Like pH, each column has a stable temperature range, and columns should not be heated above that stated temperature limit. Heating the column can decrease column pressures, improve reproducibility, and/or improve chromatographic peak shape. Consider the example on the right for a sample with 7 constituents that were analyzed at 3 different column temperatures.

Heating the column shows increased analyte separation (reduce co-elution), improved peak shapes, and shorter method times (faster gradients). Maintaining an elevated temperature can increase flow, especially when working with viscous mobile phases (e.g., isopropanol), or increase resolution and peak characteristics, especially for “sticky” samples (e.g., lipids). Both of these considerations can, in turn, decrease column pressures or even reduce method times. Stable temperatures, even if only maintaining approximate room temperature (i.e, 25 – 30 °C), increases reproducibility. Lab temperatures have been known to fluctuate by as much as 10 °C from morning to evening.