Jump to a section
General sample considerations
- Gas chromatography (GC) samples should be volatile or semi-volatile. Non-volatile samples should be analyzed by liquid chromatography (LC) instead. You can use the diagram below to help determine the best chromatographic method for your needs.
- Non-volatile samples can be derivatized to make the sample more volatile, and, thus, compatible with GC, improve the detection of semi-volatile samples, remove troublesome functional groups that might degrade the GC column, or improve thermal stability.
- Liquid GC samples should be diluted in a low boiling solvent at ca. 0.1 – 1 mg/mL.
- Gas GC samples can be procured via headspace analysis or induced via purge-and-trap methods.

Liquid sample preparation
- Samples should be dissolved in low boiling solvents.
- Applicable solvents include: benzene, toluene, chloroform, dichlormethane, ethanol, hexanes, isopropanol, methanol.
- Solvent choice depends on sample polarity, with more polar samples being dissolved in a more polar solvent (e.g., methanol) and a less polar sample being dissolved in a less polar solvent (e.g., hexanes). For samples with unknown polarity, an intermediate solvent, such as isopropanol or ethanol, may be more useful.
- High polarity solvents (e.g., methanol) should not be applied to strongly non-polar columns (e.g., polydimethylsiloxane), nor should highly non-polar solvents (e.g., hexanes) be applied to strongly polar GC columns (e.g., waxes).
- Generally, cleaner (i.e., higher grade) solvents contain fewer impurities that may interact with the polymer/sample material, interact with the column, or just complicate the analysis. There are often not GC grade solvents (GC grades are highly variable and not applicable to all solvents), so ACS or HPLC grade solvents are preferred.
- Samples are generally prepared at concentrations ca. 1 mg/mL or at 100 – 1000x dilutions. Peak shape is a strong indicator if higher or lower concentrations are needed. Broad or tailing peaks generally mean too much sample was injected and the sample should be diluted.
- Highly complex samples may benefit from additional sample preparation. Liquid (liquid phase extraction, LPE) or solid (solid phase extraction, SPE) extraction techniques can help clean and purify samples prior to analysis, reducing chromatographic complexity.
- Samples should be clear of precipitation, dust, or any other debris. Undissolved solids may require heat or sonication to assist in solvation. Additionally, filtering may be useful in further eliminating particulates. For most GC/LC applications, a 0.22 μm filter is commonly applied.
Gas sample preparation
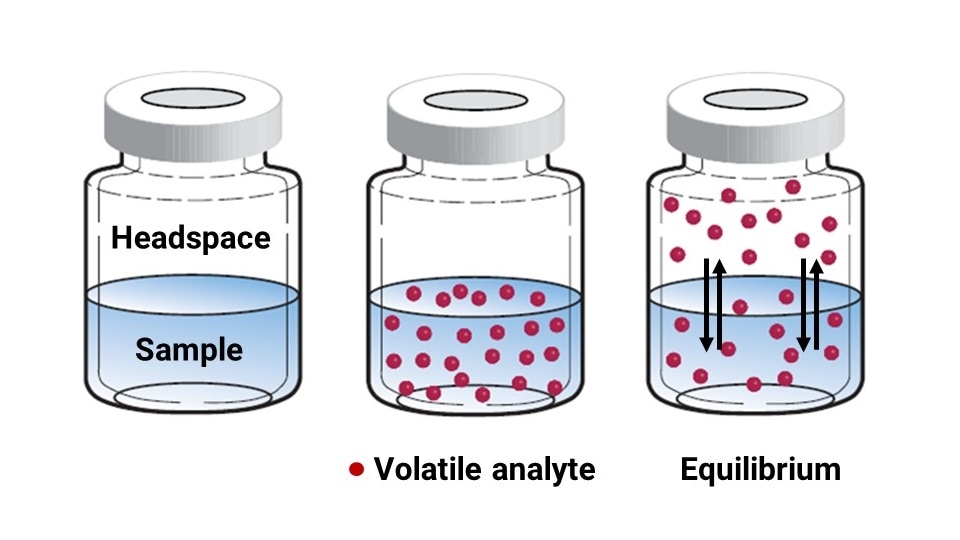
Headspace: The simplest preparation method involves holding a sealed vial at a constant temperature to allow volatile components to diffuse into the headspace of the vessel. After some time, an equilibrium is reached with some volatile analyte having entered the headspace and some remaining in solution (on in the solid material). Headspace sample preparation generally uses a larger 10 – 20 mL vial that is tightly sealed with a thick septa. A liquid or solid sample is sealed in the headspace vial and incubated in a temperature-controlled environment (e.g., oven). After some time, the gas above the liquid or solid material can be sampled via syringe and directly infused onto the column.
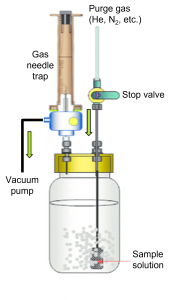
Purge-and-trap: This gas extraction method is generally performed to improve sensitivity. Instead of allowing the headspace sample to reach an equilibrium in the gas and liquid phases, a purge gas is applied through the sample to pull more volatiles into the headspace than would naturally diffuse via equilibrium processes. Here, a vacuum system is first applied to the headspace, removing any air and non-sample gases. Then, an inert gas is bubbled through the sample solution, forcing volatile sample components into the headspace. These extracted volatiles can then be trapped for GC analysis via a small trap column or gas syringe. Generally, a 100-fold sensitivity improvement can be achieved. While the purge-and-trap procedure can be performed offline, there are systems for inline purge-and-trap sampling.
Figure modified from Milton, et. al. Scientia Chromatographica. 2014, 6. 13-26. doi 10.4322/sc.2014.016.
Generally, gas samples have several benefits over liquid samples for GC analysis.
- Less sample preparation. No need to dissolve the sample in a suitable solvent or perform any extraction or derivitization to make the liquid sample more suitable towards GC analysis.
- Fewer and less abundant solvent peaks.
- Cleaner than most liquid samples, minimizing cleaning requirements (inlet, column, detector) and maintenance down-time.
- Better sensitivity and reproducibility.