Jump to a section:
Pan materials – pans are generally made of aluminum and are comprised of a pan base and lid. Different lids apply to different sample types of DSC experiments. Knowing the type of pan (basic or premium) and lid (standard, hermetic, or other) is vital as the reference pan must match the sample pan.
- Standard: solids and non-volatile sample materials.
- Hermetic: liquid or volatile sample materials. These lids are required for any samples showing >0.5% weight loss throughout the requested DSC temperature range (determined by TGA analysis prior to DSC acquisition).
- Basic/TA: nominal crafting tolerances for aluminum DSC pans.
- Premium/Tzero: crafted with tighter material and casting tolerances for better heat transfer and smaller mass differences between pans.
- Pin-hole: boiling point or vapor pressure measurements.
Non-aluminum pans may be useful for higher internal pressure ratings (> 2 atm, aluminum hermetic pans), larger or smaller sample volumes (amounts), if the sample reacts with aluminum, and/or for high temperature (> 600 °C) experiments. These pans, sample and reference, must be provided by the user.
Pans need to be well sealed prior and during the DSC experiment. The pan bottom should be flat and even (no bending or deformation). The lid should be welded to the pan bottom without holes or leakages. If the pan is leaking, discolored, or shows any residue on the pan or in the cell, the pan was not sealed appropriately (please see the User Portal for more information on how to proceed). If a pan has unexpectedly leaked during the experiment, contact the Lab Manager immediately so that cell cleaning can be performed.
A reference of an empty, sealed pan, matching the same sample pan type is necessary. If using a standard aluminum sample pan, an empty standard aluminum pan should be used as the reference. If using a premium hermetic sample pan, an empty premium hermetic aluminum pan should be used as the reference. For absolute best results, reference and sample pans should be within 0.5 mg.
Sample amounts – sample amounts are dependent on composition and/or experimental needs. Typical amounts are 5 – 10 mg. In general, larger sample sizes are needed for slower heating rates (< 10 °C/min) and smaller sample sizes are needed for faster heating rates (~20 °C/min).
Start with sample amounts between 2 – 6 mg. If your sample is known or suspected to thermally react (e.g., thermally expand), use less sample. Increase sample mass to ~10 mg if desired transitions are weak. Sample >10 mg should be avoided. If desired transitions are still weak, consider a modulated experiment (i.e., mDSC).
| Organic | 2 – 10 mg |
| Inorganic | 5 – 50 mg |
| Strongly exothermic | < 5 mg |
| Purity or kinetics | 1 – 5 mg |
| Metal or Chemical melting point | < 5 mg |
| Polymer glass transitions or melting point | ~10 mg |
| Composit or blend | > 10 mg |
| Heat capacity | ~10 mg |
| Modulation (mDSC) | 2 – 10 mg |
Use more sample if (1) transitions are weak, (2) filled or dilute, or (3) low heating rates are used.
Use less sample if high heating rates are used.
Sample materials – solids and film materials are preferred, but liquids and other solid forms are applicable. Samples should be representative of the bulk material. Mixing or composite methods should be applied to ensure bulk similarity to the analyzed sample.
The sample should be thermally stable throughout the DSC temperature range. To ensure the sample is not actively decomposing during the DSC experiment, a TGA analysis is required before running the sample by DSC. From the TGA, acquire the T95 temperature (i.e., the temperature at which < 5% of the sample has decomposed). The maximum DSC temperature should be set at T95 – 10 °C (i.e., 10 °C before the sample’s T95). NO DSC measurements should be conducted above the sample’s T95.
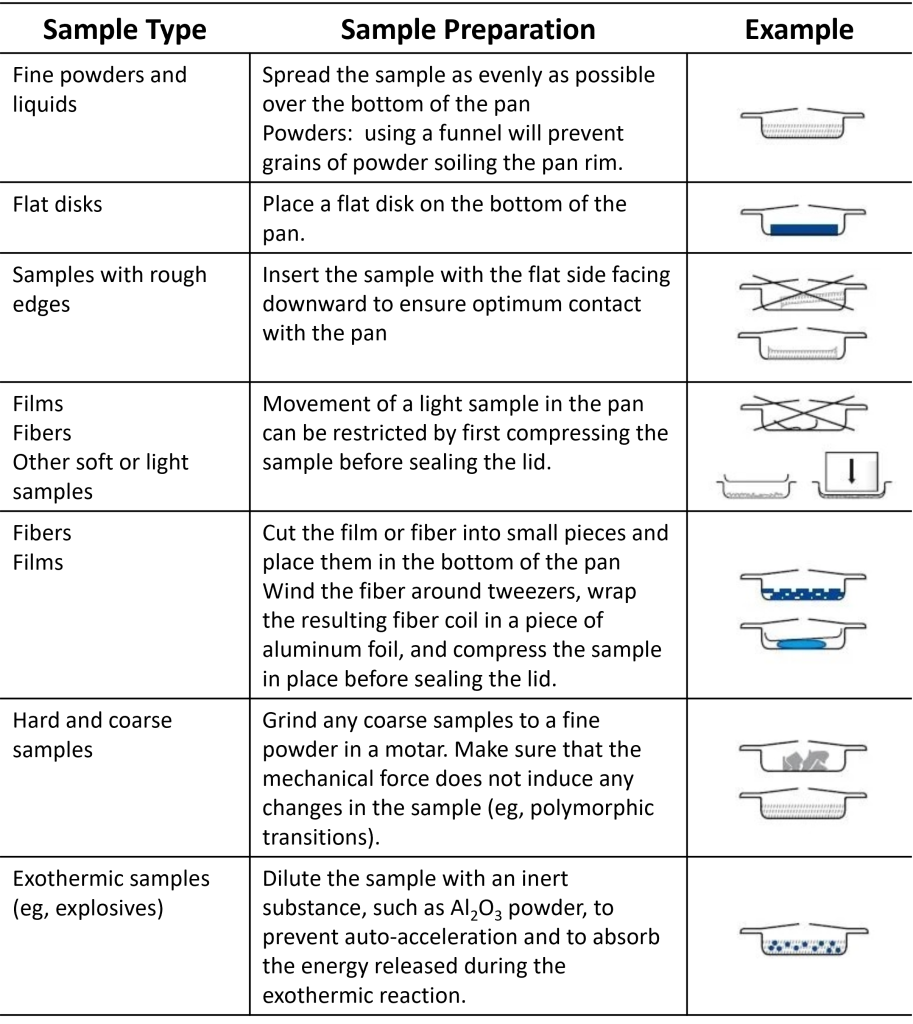
Good DSC measurements rely on good thermal contact at all contact points–cell pestle-pan bottom, pan bottom-sample, and sample-pan lid/top. To minimize the thermal gradient within the sample and pan, ensure (1) a flat, non-deformed pan bottom, (2) packed or flat sample, (3) orient the lid for best thermal contact with the sample (i.e., TA Instrument’s pans are designed to be sealed concave or “bump” up, away from the sample), and (4) only half fill a pan with sample. Use these suggestions depending on your specific sample type for best results:
- Cut the material if possible. Crushing the sample is not recommended.
- Cover as much of the pan bottom as possible.
- To achieve good heat transfer between the sample and pan, the sample should have one surface with an area as smooth as possible that allows the sample to lie flat on the pan bottom.
- Compacting powdered or fibrous material may be necessary to ensure the sample height is not too great.
- The pan bottom should be flat. Deformation of the pan will introduce a thermal gradient within the sample that will negatively impact the data.
- If analyzing a metal, a high temperature ramp should be performed initially to first melt the sample.
- Do not overfill a pan as this may cause the sample to leak out, buckle the seal, or explode the pan. Only fill the pan approximately half. If your sample is known or thought to thermally expand, fill less.
For detailed DSC sample preparation instructions, visit the Sample Preparation page in the User Portal.
Special considerations or experiments
For any of these experiments, please contact the Lab Manager directly to discuss specific experimental and sample preparation requirements as well as coordinate instrumentation time.
- Explosive materials – only small amounts of explosive materials should be used. Material should be combined with an excess of inert material, such as aluminum oxide powder. Any samples known or suspected to be explosive hazards should be explicitly stated as such on the submission form.
- Heat capacity – measurements are generally slower and over shorter temperature ranges. Heat capacity measurements are acquired in comparison to a sapphire (preferred) or polystyrene standard, acquired under the same conditions and method as the sample. Calibration for these experiments are short-lived and should be performed within the day (preferred) of sample acquisition/measurements. A sample mass of ~10 mg is highly recommended for best results. There are 3 methods for acquiring heat capacity data, in increasing accuracy:
- Direct Cp (fastest) – A single sample Cp acquisition after a single sapphire Cp calibration. Several samples can be acquired within the day of calibration, without any additional sapphire calibration. Pans must be premium (Tzero) hermetic.
- Three run Cp/ASTM method (traditional) – Cp data is acquired as a set of 3 runs: baseline, sapphire, and sample run. Pan type (premium/Tzero hermetic) and mass (53.9 mg) must be identical for all 4 pans: reference, baseline sample, sapphire, and sample. No additional calibration is required, as all samples contain their own calibration from the baseline and sapphire runs acquired immediately before the sample run.
- Modulated Cp (most accurate) – Sample Cp acquisition following a reversing (via mDSC) sapphire Cp calibration. mDSC measurements are slow (< 3 °C/min), so a very narrow temperature ramp is recommended. Several samples can be acquired within 1-2 days of calibration, without any additional sapphire calibration. Pans must be premium (Tzero) hermetic.
- Temperature sensitive experiments – pans are typically loaded at 40 °C; however, for some temperature sensitive samples, loading temperatures can be lowered to 20 °C, upon request. For these experiments, samples are recommended to be prepared and crimped (pan) in a refrigerated environment. Prepared samples should be stored cold until ready for analysis.
- Contact Dr. Bailey for access to a cold room, LEI 420.
- Alternate atmosphere analysis – DSC cell is purged and acquired in nitrogen. Alternate acquisition gases (helium, nitrogen, air, argon) may be used upon request, or multiple gases may be introduced (2 gas maximum) during the experiment. For these experiments, unsealed pans (sample and reference) may be used.
- Modulation – if sample amounts are limited or transitions are small. Some applications include:
- complex transitions, involving multiple processes
- limited sensitivity
- limited resolution
- heat capacity
- thermal conductivity