Jump to a section
Material
- Samples need to be liquids, either dispersed in solvent or emulsions.
- Solid or dried samples are not directly compatible with this measurement.
- A 1 – 2 mL volume is recommended; however, for volume limited samples, 100 – 200 µL may be acceptable.
- Handle all materials with powder free gloves to ensure no glove-based contamination.
- Make sure all solvents and materials are cleaned and/or filtered to remove any dust or other interfering particulate contaminates.
- Clean all materials with deionized (DI) water. A puff of compressed air can blow out residual dust and/or solvent. Including: pipettes, sample containers, cuvettes, solvents, etc.
- It is especially important to filter salted solvents prior to application (after mixing the solvent and salt material).
- Filters 0.1 – 0.2 µm can be used on pure or prepared solvents, prior to combining with sample.
- Filters 0.2 – 0.45 µm are usually suitable for prepared samples.
- Ensure the filter/pore size chosen will pass the desired sample material (pore size ~ 3x largest sample particle size).
- Always rinse the filter prior to use.
- Prior to acquiring data, incubate the sample and cuvette in the instrument’s sample holder for 10 – 15 minutes to ensure thermal equilibrium.
- If just turning on the instrument, ensure at least 30 min [laser] warm-up and stabilization prior to taking measurements.
Solvent
- Solvent must be non-reactive with the sample. Solvent should not dissolve the materials of interest.
- Pure solvents (ACS or HPLC grade), free of possibly interfering contaminates and/or dust, are preferred. Impure solvents can increase the measurement noise, particularly for small or weakly scattering sample particles.
- The most common solvents are:
- Water (do not use DI water unless specifically adding salt and/or surfactant)
- Methanol
- Ethanol
- Toluene
- Glycerol
- Solvents such as toluene and DMSO (and similar) can interfere with the measurement itself (e.g., solvent scattering increases background noise, viscosity changes with temperature) and should be avoided.
- Surfactant might be useful to aid dispersion, especially for solid sample material in water.
- Prepare dilute surfactant, 1 – 10%. Mix a drop of diluted surfactant with the solid sample and then add water.
- Only use a few drops of diluted surfactant.
- Common non-ionic surfactants:
- Triton X-100
- Igepal CA-630
- TWEEN 20 or TWEEN 80
- Span 20 or Span 80

DLS Sample Preparation, Entegris technical note.
- For charged particle samples or aqueous suspensions, salts can reduce interfering particle interactions. Salt concentrations range between 0.1 – 10 mM (10 mM is most common). Choose salts based on solvent:
- Aqueous: KBr, NaBr, KNO3
- Nonhalogenated organics (e.g., THF, DMF, DMA): LiBr
- Halogenated organics (e.g., CHCl3, CH2Cl2, o-dichlorobenzene): NBut4Br
Concentration
- 0.1 – 1% or 1 – 10 mg/mL sample concentrations are most common.
- Sample should be clear or mildly hazy.
- Verify there is no sample precipitation. Precipitation is indicative of an overly concentrated sample (dilute) or a needed dispersion agent (add salt or surfactant).
- Colored samples may be acceptable, provided no sample absorption occurs. In general, colored or fluorescing samples are harder to measure and should be avoided, if possible.
- For white, darkly colored, or very hazy samples, additional dilution will be necessary.
- To determine if coloration is from over-concentration or natural coloring, try reading text through the sample. If text can still be read, coloration will likely not affect measurements (provided sample will not absorb laser light).
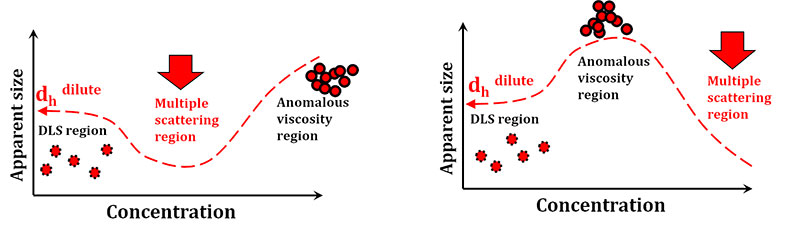
- Optimal concentration is at the hydrodynamic radius plateau or constant region (see figure below). Sample should be diluted and measured until no change in hydrodynamic radius is observed.
- Highly concentrated samples may result in underestimation of hydrodynamic radius from multiple scattering.
- After measurement, dilute the sample 50% and re-measure. If the resultant size is the same and the count rate is 1/2 as previous, the first measurement (previous concentration) was acceptable.
- Suspensions should be well dispersed and homogenous.
- Polymers, proteins, and other fragile sample types should be gently mixed. Allow 12 – 24 hours dissolution time. Swirling or pipette mixing can be used as a mixing aid. Do not sonicate.
- For sturdy samples, vortexing or sonication (bath only) for 15 minutes can assist homogenous distribution.
Zeta potential (ZP)
Also called the electrokinetic potential. A measure potential difference between electrophoretically mobile particles and the dispersant [surrounding those particles] at the slipping plane. As the electrophoretic particle motion also scatters incident light, both particle size and zeta potential can be measured/calculated simultaneously via DLS.

- As electrokinetic potential is highly dependent on sample environment, ZP samples should be carefully prepared/diluted with the original sample solvent.
- If possible, procure supernatant from the original concentrated sample, either by filtering or centrifuging. Use supernatant for dilution.
- Allow the concentrated sample to naturally sediment and measure the resulting supernatant.
- Attempt to recreate the original medium as closely as possible, accounting for pH, total ionic concentration, concentration of any known additives, etc.
- Do not dilute ZP samples with DI water, unless the original sample itself was prepared in DI water.
- The addition of any surfactants, salts, etc. necessary for sample dispersing (as described above) will alter the chemistry from the original ZP sample. The ZP of the original sample will be different from the ZP of this DI diluted sample.
- ZP samples diluted in DI water (without any added salts, etc.) have poor reproducibility.
- DI water has a low conductivity, making the electrical double layer difficult to measure and resultant ZP measurement difficult to interpret.
- If diluting ZP samples in polar or non-polar solvents, the lack on ions can lead to over-estimation of the measured ZP.
Cuvette
- Before using, be sure to rinse cuvettes with DI water to remove any residual dust.
- Invert cuvette and blow compressed air to push out remaining dust and/or water before filling with sample.
- Use caution if using metal tips as this can damage the cuvette.
- Ensure there are no bubbles when filling the cuvette.
- Wipe cuvette outside with optical tissue.
- Cover cuvette top, use cuvette lid, to ensure no dust or debris contaminates prepared sample.
| Cuvette type/material | Temperature range | Solvent compatibility | Experiment compatibility |
| Square, polystyrene (disposable) | 0 – 70 °C | Aqueous, weak polar | DLS only |
| Micro-cuvette, plastic | 0 – 70 °C | Aqueous, acetone, benzaldehyde, butanone, dioxane, DMF, ethyl acetate, isopropanol, various acids and bases | DLS: 40 µL min. |
| Micro-cuvette, quartz | 0 – 120 °C | any solvent | DLS: 12 – 45 µL |
| Square, glass | 0 – 120 °C | any solvent | DLS only |
| Folded capillary zeta cell, disposable/plastic | 0 – 70 °C | aqueous, weak polar | DLS; pH titration; Zeta Potential |
| High concentration zeta potential cell | 0 – 70 °C | aqueous, weak polar | DLS; pH titration; Zeta Potential: high concentration (e.g., ceramics, cosmetics, emulsions), low volume samples |
| Universal ‘Dip’ cell | 0 – 70 °C | aqueous, non-polar* | Zeta potential |